Abstract
Severe acute respiratory syndrome coronavirus (SARS-CoV-2) has emerged as a pandemic and named as novel coronavirus disease (nCOVID-19). SARS-CoV-2 is different from other known viruses due to multiple mutations on the sites of nonstructural proteins (NSP) 2 and 3, and the varying nature of virulence between different persons. Immunotherapies such as vaccines and monoclonal antibodies have a protective effect on the patients bringing them to the front of the line of potential treatments. The present review intends to cover the development of 20 different vaccine candidates categorized under live attenuated vaccines, inactivated vaccines, subunit vaccines, viral vector-based vaccines, and nucleic acid vaccines. Formulation of these vaccine candidates by various companies in collaboration with global organizations and their status of clinical trials were addressed. On the other hand, various approaches for post-vaccination surveillance using nucleic acid and protein biomarkers imbued on suitable platforms were also highlighted to sum up the immune therapeutics for Covid-19.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronaviruses (CoVs) are single-stranded RNA (+ssRNA) viruses that fall in the coronaviridae family under subfamily coronavirinae. They are classified into alpha, beta, gamma, and delta coronaviruses. Generally, its infection causes symptoms like common cold to severe illness in animals and humans (Ahmed et al. 2020; Ramaiah and Arumugaswami 2020). In 2002, betacoronavirus was found responsible for causing respiratory infection for Chinese people, and based on the symptoms Epidemic Disease Center (EDC) has named the disease a Severe Acute Respiratory Syndrome (SARS). Later the Middle East respiratory syndrome (MERS) was reported in Saudi Arabia with similar symptoms caused by another strain of betacoronavirus (Lau and Chan 2015; Ramaiah and Arumugaswami 2020). Common symptoms reported in humans during both the conditions were fever, nonproductive cough, headache, progressive respiratory failure (Dhama et al. 2020). Intermittently, genetic mutations and recombination rates are high in coronaviruses and these mutated viruses pass from animals to animals and habitat to new hosts had a major impact on the spreading of disease worldwide in humans and animals (Lau and Chan 2015). Recently in December 2019, similar symptoms like SARS were reported in the city of Wuhan, China for elderly persons or people with premedical conditions. Based on the causative virus for symptoms and year of origin, the disease was referred as coronavirus disease-2019 (COVID-19). Based on its phylogenetic relationship severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is categorized as betacoronavirus which causes COVID-19 (Ahmed et al. 2020).
Like all other viruses, coronaviruses are also circulating in nature for the past couple of decades and alpha/ beta-coronaviruses are being transmitted from animals and affecting humans. Among these, some strains are sporadic, and others are capable of causing an epidemic. Zhou et al. (2020) confirmed Bat as the natural host reservoir for SARS-CoV-2 along with other coronaviruses after performing genome sequencing which showed 96.2% similarity with the genome of bat CoV RaTG13. Phylogenetic analysis showed similar residues of receptors suggesting the same ancestral connection between these two viruses and the possibility of having turtles, pangolin, and snakes as intermediate hosts (Liu et al. 2020). Transmission of the virus to humans must have happened due to consumption or direct contact with intermediate/reservoir host animals. On the other hand, human to human transmission occurred between closely associated people through the unintentional spreading of viral droplets by respiratory secretion during coughing and sneezing, which are the common symptoms (Guan et al. 2020).
Wu and his team (2020) isolated SARS-COV-2 strain from an COVID-19 infected patient and studied its complete genome. The genomic structure is organized as +ssRNA containing ~ 30 kilobases in length with a 5′-cap structure and 3′poly-A tail. Further, the viral genome encodes for 4 structural proteins-membrane (M), spike (S), nucleocapsid (N), and envelope (E) required for producing viral particles supported by 16 nonstructural proteins (NSPs) (Chan et al. 2020). Angeletti et al. (2020) found a mutation on NSP 2 & 3 which were actually responsible for the enhancement of SARS-COV-2 virulence and made the researchers consider it as a novel coronavirus (nCOVID-19). At present, there is no specific drug available for the treatment of this virus but broad-spectrum anti-viral drugs are showing some promising results (Rothan and Byrareddy 2020). The most frequently used anti-viral drugs throughout the world include chloroquine, hydroxychloroquine, lopinavir/ritonavir, favipiravir, and remdesivir. The World health organization (WHO) is launching “Solidarity” clinical trial to evaluate hydroxychloroquine/chloroquine, remdesivir, and lopinavir-ritonavir with and without interferon beta for treating COVID-19 (WHO 2020a). Wang et al. (2020a) evaluated the antiviral efficiency of ribavirin, nitazoxanide, penciclovir, chloroquine, nafamostat, favipiravir, and remdesivir against a clinical isolate of 2019-nCoV in vitro and revealed the efficiency of remdesivir and chloroquine in controlling Covid-19. Besides, Dang et al (2020) reported that the two drugs hydroxychloroquine and remdesivir, which were previously used for other indications should be repurposed to treat Covid-19. Owing to the safety, cost, efficacy, and ethical concerns, these two drugs have received emergency use authorisation by the US Food and Drug Administration. Gao et al. (2020a, b) evaluated computed tomography (CT) of COVID-19 patients and reported the efficiency of hydroxychloroquine in reducing respiratory symptoms and pulmonary inflammation. Moreover, the patients showed nasopharyngeal clearance of the virus on 6th day of receiving hydroxychloroquine /azithromycin drug (Gautret et al. 2020). Further, Wang et al. (2020a, b, c) reported that remdesivir was superior to placebo in reducing the recovery time in Covid-19 patients by lowering the respiratory tract infection.
Convalescent plasma (CP) therapy is well known for two decades and was successful in treating SARS, MERS and H1N1. As these viruses share similar virological and clinical characteristics with COVID-19 the researchers strongly believe that CP therapy can become a promising rescue for COVID-19 infected patients (Chen et al. 2020a). Nevertheless, the Indian Council of Medical Research (ICMR) stated clearly that plasma therapy was not approved for COVID-19. Therefore, the researchers are working on the development of vaccines to prevent everyone from getting infected.
SARS-CoV-2 vaccine platforms and their clinical trails
The Covid-19 pandemic had a drastic effect on lives as well as on the global economy. Governments across the world are encountering with recession and high mortality, and the vaccine is a conclusive solution to rescue mankind and regain their normal livelihood. Vaccine development is an extensive process with high chances of failure and even involves numerous challenges and safety issues to get accepted and approved. Various stages involved in the development of new vaccine along with the timeline have been incorporated in Table 1 (Janani and Venkatesh 2019; Gao et al. 2020a, b).
Besides, the roadblocks in vaccine development and commercialization process are enumerated below.
-
Extensive clinical trials on various animal models and humans before licensing.
-
Mandatory partnerships of small vaccine developers with large manufacturers for the development of vaccine from proof-of-principle to the stage of commercialization.
-
Thorough testing of vaccines to meet all the necessary criteria.
-
Constraints in the vaccine production technology.
-
Vaccine cost, license agreements, and vaccination policies.
Should be handled properly to ramp up the production and supply of the vaccine to the global market to halt the spread of SARS-COV-2.
With the knowledge gained from SARS and MERS vaccines development path, multiple research teams are working on various forms of SARS-COV-2 vaccines such as inactivated, live attenuated, viral vector, protein subunit, and nucleic acid (DNA and RNA) vaccines to release potent vaccines to check the mortality rate.
Inactivated vaccines
Purified inactivated viruses have been used traditionally for vaccine development (Vellozzi et al. 2009). The SARS-COV-2 is inactivated either by using chemical agents such as β-propiolactone, formaldehyde, glutaraldehyde, or by physical agents like heat (65 °C), ultraviolet light (254 nm), and high alkaline and acidic conditions (Le et al. 2020). Though these inactivated viral vaccines are preferred due to their safety reasons, some problems are encountered due to their usage. In some cases, the administration of these vaccines may render incomplete protection and poor neutralizing antibody response due to the obliteration of viral neutralizing epitopes. Moreover, a huge quantity of viruses is required for the production of this vaccine (Menachery et al. 2018).
The whole virus vaccine stimulates toll-like receptors (TLR) such as TLR 3, TLR 7/8, and TLR 9 which causes inherent immunogenicity. Serum Institute of India (SII) in collaboration with Codagenix, a US-based biotechnology company has developed a synthetic inactivated virus to develop the vaccine candidate which is presently under animal trials (WHO 2020b). In China, Wuhan Institute of Biological Products (WIBP) along with the Wuhan Institute of Virology (WIV) developed an inactivated COVID -19 vaccine which is in phase II clinical trials (WHO 2020b). Another inactivated vaccine candidate, PiCoVacc, developed by Sinovac Biotech, Beijing when tested upon mice, rats, and rhesus macaques induced neutralizing antibodies in all the animal models (WHO 2020b).
Live-attenuated vaccines
The original SARS-Cov-2 is weakened by passing through animal or human cells until it picks up mutations. The weakened SARS-Cov-2 with altered genetic code is used for vaccine development (Gao et al. 2020a, b). This strategy can induce a quick and strong immune response and can scale up for mass production. The creation of infectious clones for attenuated coronavirus vaccine is tedious and consumes more time due to its large genome size. This vaccine can be dangerous for immune-suppressed people and the production process of vaccines involves extensive safety testing (Kim et al. 2020).
Codagenix, the USA in collaboration with Serum Institute of India is working to weaken SARS-CoV-2 and produce a vaccine (CDX-CoV) containing all proteins from the natural virus (Shieber 2020). Besides spike protein, the vaccine will target the structural and non-structural proteins of SARS-CoV-2. This vaccine is still in the pre-clinical stage. Another live attenuated vaccine for Covid-19 is being developed by Griffith University, Australia in collaboration with Indian Immunologicals (IIL), India. This team is trying to use codon de-optimization technology for genome alterations to reduce the replication efficiency of the virus in human cells (Pagliusi et al. 2020). The developed vaccine will mimic the infection, stimulate all the arms of the immune response without causing any disease. This vaccine can render lifelong immunity against SARS-CoV-2 with a single immunization.
Viral vector-based vaccines
These vaccines offer long-term stability, a high level of protein expression, and a strong immune response (Le et al. 2020). Virus-like particles (VLPs) are suitable vectors for gene delivery because they mimic the native virions (Swadling et al. 2013). There are two approaches to a viral vector vaccine, replicating viral vector, and the non-replicating viral vector. Various replicating viral vectors in use are the measles virus, adenovirus, poxvirus, and vesicular stomatitis virus (Humphreys and Sebastian 2018). Because of their non-pathogenicity in humans, the coding sequence required for protein/subunit antigen is attached to the viral genome for optimal expression, assembly, and packaging. Due to their replicating nature, these viral vectors elicit a complete immune response at a lower dosage of 3–4 logs.
Owing to its capability to infect the antigen-presenting cells, measles virus-based vectors are considered for vaccine preparation against SARS (Zuniga et al. 2007). In a non-replicating viral vector, the genes essential for replication is replaced with antigenic protein-coding genes (Robert-Guroff 2007). Apart from accommodating large gene inserts, these vectors provide stability by not regaining the virulence when compared to replicating viral vector. The viral vector-based vaccines can increase immunogenicity without an adjuvant and promote a vigorous cytotoxic T cell response to remove virus-infected cells (Lu 2020). Adenoviral vectors are best characterized by viral vectors and probably most potent at T-cell priming in non-human primates and humans (Swadling et al. 2013).
One vaccine candidate, ChAdOx1 nCoV-19 is made from a genetically modified version of adenovirus, which does not replicate in humans. When vaccinated into the body, it will give an immune response and prevents SARS-CoV-2 from entering the human cell. Oxford University has completed the phase-2 trial and moving towards phase-3. Another vaccine candidate for Covid-19 was developed by CanSino Biologics, China in collaboration with the National Research Council (NRC), Canada. This vaccine is a genetically engineered vaccine candidate with the replication-defective adenovirus type 5 as the vector, to express SARS-CoV-2 spike (S) protein. Phase II clinical trials of this vaccine were successfully completed in more than 100 human subjects (WHO 2020b).
One more vaccine candidate, Ad26 for SARS-CoV-2 uses the same technology involved in Janssen’s Ebola vaccine. Non-replicating adenovirus 26 (Ad26) vector carrying undisclosed genetic material of SARS-CoV-2 is administered through the intranasal route (Buonaguro et al. 2020). The clinical trials for the Ad26 vaccine are set for fall 2020. Janssen Pharmaceutical Company with the support of Biomedical Advanced Research and Development Authority (BARDA) has planned to scale up this vaccine to 300 million doses/ year.
Subunit vaccines
Subunit vaccine contains only antigenic parts of the pathogen which is required to elicit an immune response. These vaccines give partial protection against the virus as they have a very poor track record of stimulating humoral immunity and neglecting cell-mediated response (Jiang et al. 2012). The majority of the subunit vaccine candidates were developed against whole or fraction (N-terminal domain or receptor-binding domain) of spikes available on the surface of SARS-CoV-2. Recently developed or established adjuvants are being used along with the fraction of the spike protein for enhancing the immune response (Chen et al. 2020b). Research is being made for the development of cocktail vaccines by using additional viral antigens like nucleoproteins, non-structural proteins, etc. A vaccine candidate XWG-03, based on multiple truncated spike proteins has been developed by Innovax Biotech in association with Xiamen University. GlaxoSmithKline (GSK) collaborated with the work and it is offering adjuvant for preclinical trials of the vaccine candidate. The series of spike proteins will be evaluated and a lead candidate will be established based upon the immunogenicity data (WHO 2020b).
Another vaccine candidate NVX-CoV2373, a pre-fusion protein vaccine was prepared by using nanoparticle technology and Matrix-M adjuvant of Novavax, Maryland based company to stimulate the immune system and antibody production. Besides, COVID-19 S-Trimer, another protein-based corona vaccine was developed by Clover Biopharmaceuticals, China in collaboration with GSK which will provide its adjuvant system for evaluation of S-Trimer. Further, Clover will scale-up the production of this vaccine (WHO 2020b). One more subunit vacacine was being developed with the collaborative efforts of the Indian Institute of Science (IISc) and Mynvax, a Bengaluru based startup. Mynvax has already developed several candidate immunogens which are now moving towards animal trails. Moreover, this firm has applied to BIRAC for a grant of Rs. 15 crores for scaling up the process.
Nucleic acid vaccines
The nucleic acid-based vaccine is a new approach of immunization in which either DNA or RNA containing viral genes is injected into the animal to express the encoded protein and develop both humoral or cell-mediated immunity. The advantages of these vaccines are easy delivery and expression of the protein in less duration. Multiple DNA vaccines have been approved for animals but in-depth studies on the safety, feasibility, and efficacy of these vaccines are required for human application.
DNA vaccines
INOVIO Pharmaceuticals, an American-based company developed a synthetic vaccine candidate INO-4800 (DNA vaccine) which targets spike protein of coronavirus. During pre-clinical studies, they have immunized mice and guinea pigs with INO-4800 and evaluated the production of functional antibodies, antigen-specific T cell responses, and distribution of SARS-CoV-2 specific antibody to the lungs. The study proved INO-4800 as an efficient vaccine candidate for COVID-19. With this input, INOVIO developed a smart device CELLECTRA, which uses an electric pulse to open the pores in the human cell to deliver the DNA plasmid. The DNA will get translated into the cell and produce proteins that activate the immune system to generate T cells. INOVIO expanded DNA-encoded monoclonal antibody technology for enhancing the therapeutic range of monoclonal antibody and successfully entered into phase I clinical trials (Smith et al. 2020).
RNA vaccines
mRNA sequence that has the potential to trigger the immune response by encoding disease-specific antigen is introduced into the human body through various delivery platforms viz., lipid nanoparticles (LNPs), lipoplexes, and polyplexes (Chen et al. 2020b). This vaccine can be produced at an affordable cost that has a wide scope for scale up to meet the high demand of the pandemic. RNA vaccines are safe in comparison to other vaccines and elicit high immunogenicity.
mRNA-based vaccine candidates for Covid-19 are showing fast progress. Moderna, an American biotech company stood first in developing and testing the mRNA vaccine (mRNA-1273) in humans. mRNA-1273 is expected to enter into Phase II clinical trials in the second quarter of 2020, based upon the outcome of Phase I trials. Another m-RNA based vaccine BNT162, developed by Germany-based BioNTech in partnership with Pfizer, New York received approval from the regulatory authority to start Phase I/II clinical studies. BNT162 vaccine candidates will be tested in 200 volunteers for determining the optimal dose and evaluating the immunogenicity and safety of the vaccine (WHO 2020b).
As vaccine development for SARS-CoV-2 is in its infancy, a complete picture of the vaccine platform is not yet framed. The governments of various countries like the United States, United Kingdom, China, Germany, Japan, India etc. are working dedicatedly in collaboration with international institutions, private sectors, research institutions, and nonprofit organizations across the globe, to combat Covid-19. Approximately, 34 candidate vaccines are in clinical evaluation and 142 are in preclinical evaluation (WHO 2020b). A shortlist of several vaccine candidates and their current status of clinical trials have been provided in Table 2.
Collaborative global efforts are in progress to control the COVID-19 pandemic (Table 2). Zhu et al. (2020) reported that both humoral and T cells immune responses were developed in healthy individuals (n = 108) between the age group 18–60 within 14 days of administration of a single dose of Ad5-nCoV. The participants were segregated into three groups viz., high dose group (n = 36), medium-dose group (n = 36), and low dose group (n = 36). After 28 days, 28/36 (78%) of the low dose group, 33/36 (92%) of the medium-dose group, and 36/36 (100%) of the high dose group developed detectable immune response (Zhu et al. 2020). There were no serious adverse events but several symptoms like fever (46%), fatigue (44%), headache (39%), and muscle pain (17%) were observed. To evaluate the safety and efficacy of the vaccine (Ad5nCoV), a phase two trial was launched in Wuhan, China, with n = 500 healthy adults, which are divided into low dose (n = 125), middle dose (n = 250), and control (placebo, n = 125). Similarly, Moderna also initiated a phase I trial by giving two doses of vaccine, 25 µg (mcg) and 100 mcg to the participants (n = 105) of age groups 18–55. The neutralized Abs level was higher in 100 mcg administered participants than recovered patients. Moderna has got FDA approval for phase II and even initiated phase III trials.
AstraZeneca started phase I/II trial of AZD1222 (formerly known as ChAdOx1 nCoV-19) in May 2020 enrolling 1000 adults of 18–55 years (AstraZeneca 2020). Researchers are currently working with viral DNA, mRNA, micro genes vaccines, and trying to identify effective vaccines and therapeutics for controlling the pandemic.
Design and development of theranostic vaccines
Theranostics is a multidisciplinary approach developed a combination of therapeutic and diagnostic agents in major to examine the response of treatment and optimize drug selection to strengthen the personalized healthcare system (Fig. 1). This approach enhances the drug efficacy by avoiding unnecessary usage of alternative medication resulting in saving lives with cost-effective practices (Bartlett et al. 2012). This approach is being used in vaccine and cancer research with a possibility to identify and track immune cells that are amended by immune stimulants at the cellular level during vaccination (Karabin et al. 2018).
The immune system is a complex system with a receptive network of bioactive molecules that must uphold homeostasis inside the host body and evolve continuously to fight against invading pathogens (Miller et al. 2019). In addition to its dynamic nature, the uniqueness of persons varying from age, gender, food habits, lifestyle, earlier health history, and prior exposure to certain immuno-stimulators play a major role in influencing the response to stimuli (Benne et al. 2016). A similar pattern is being noticed in COVID-19 patients and a lot of parameters need to be considered by researchers before developing a suitable vaccine.
Moreover, several new cases/deaths increasing day by day and these changes in the viral genome due to mutations have created a lot of hype among electronic and print media. This kind of publicity is also supported by available literature on frequent genomic changes in various RNA viruses such as Ebola, rabies, measles, flu, etc. (Holwerda et al. 2020). The speculation of mutant strains of COVID-19 rose to almost 200 varieties and several researchers from various industries, institutes, and research labs are working in this line to confirm the polymorphism.
Using full-length genomic sequences submitted in National Center for Biotechnology Information (NCBI) and Global Initiative on Sharing All Influenza Data (GISAID) databases, Wang et al. (2020a, b, c) have analyzed homology in the sequences of 95 strains of SARAS‐CoV‐2. Even though 13 variation sites in the regions of 1b, S, 3a, M, 8, & N were observed, the similarity among 95 strains was 99.9% at the nucleotide level and 99.8% at the amino acid level. They were successful in recognizing the positions nt28144 in the open reading frame (ORF) 8 and nt8782 in ORF 1a with a ~ 30% mutation rate. Shen et al. (2020) compared genomic changes among three varieties of samples collected from bronchoalveolar lavage fluid of SARS–CoV-2 infected patients, community-acquired pneumonia patients, and healthy controls. Metatranscriptome sequencing of these samples revealed a median number of intrahost variants between 1 and 4 in COVID patients and ranged from 0 to 51 in remaining samples confirming limited diversity in polymorphism data.
In this context of growing health emergencies and lack of sufficient information on mutated coronaviruses creating concern among researchers on the development of a universal vaccine. This problem can be resolved by designing a theranostic vaccine instead of a single shot to prevent multiple strains. Even though this concept is not established at the moment, through scientific exploration and expansion in these lines can bring the developed and developing countries on to the same platform due to its cost-effective and precise mechanisms.
Risk assessment of laboratory testing
Either it is a regular vaccine or futuristic personalized theranostic vaccine, always the risk is involved in the design and development process. Preventive steps for risk assessments before laboratory testing play a key role in detecting/preventing errors at pre-analytical, analytical, and post-analytical phases (Chávez 2019). Clinical Laboratory Standards Institute (CLSI) an international organization provides a complete set of guidelines for all the laboratories to identify and mitigate risk management for all the stakeholders involved in the process (Njoroge and Nichols 2014). Centers for Disease Control and Prevention (CDC) on the other hand have issued specific guidelines on biosafety levels (BSL) based on the exposure to infectious/biohazardous agents. There are four levels of biosafety based on the level of containment controls to protect workers, the environment, and the public (Ta et al. 2019). The risk assessment team must carefully identify all potential scenarios/threats that could lead to a negative outcome and chalk out a plan to resolve such threats (Fig. 2) (Hao et al. 2017).
A couple of safety measures taken during the design/ production of SARS and MERS-CoV vaccines can be used as a reference and out of which the following has a lot of significance.
-
i.
Ensuring safety to humans: Avoiding all kinds of complications or side effects due to vaccination during formulation.Ensuring safety to humans: Avoiding all kinds of complications or side effects due to vaccination during formulation.
-
ii.
Providing long term protection: Single-shot vaccine should provide lifelong immunity.
-
iii.
The risk for aged people: In general, vaccines are less immunogenic in elderly people (≥ 50) compared to the youth. Higher antigen dose/ alternative routes for administration/ usage of adjuvants can overcome the problem in elderly personnel (Wienberger 2018).
-
iv.
Fever: Body temperatures above 100.4 ºF can be an expression for infection and that can bring off a poor response on the vaccine and lead to negative immune response ontohe infectionFever: Body temperatures above 100.4 ºF can be an expression for infection and that can bring off a poor response on the vaccine and lead to negative immune response ontohe infection.
-
v.
Pregnant women, immune-suppressed patients, and cytostatic patients: Should not be given attenuated vaccines due to unavoidable complications (Nordeng et al. 2010).
Besides these general guidelines, considering the virulence and contagious nature of SARS-CoV-2; CDC recommended usage of unidirectional air-flow BSL-2 facility for non-propagation diagnostic laboratory work. Propagation studies like virus culture, isolation, or neutralization assays should be performed with BSL-3 precautions and procedures (Iwen et al. 2020). Another mysterious risk related to COVID-19 is variations in the severity of cases observed in China during December 2019 and the remaining parts of the world. Tetro (2020) suggested the disparities are due to the prior exposure of that individual with the coronavirus and can be confirmed by assessing the antibody-dependent enhancement (ADE).
Post vaccination surveillance
Vaccination is the most successful and cost-effective tool for the control or eradication of viral diseases. It is a combination of several steps including immunization, surveillance of infected people before/after vaccination, and pruning of host susceptibility towards infection. Surveillance is one of the significant phase in controlling viral diseases, which can be achieved either by active or passive approaches and in most of the cases both were conducted. Community-based surveillance is considered as passive surveillance mainly done by taking feedback from local people and active surveillance activities include monitoring of patient directly (Karimuribo et al. 2017). However, active surveillance is a costly process and requires substantial human and laboratory resources. Post surveillance is very important because, the very nature of biological products is dynamic and lead to some unexpected adverse risk such as anaphylaxis, vaccine-strain systemic infection and vaccine-associated paralytic condition (Corman and Landt 2020). As per the available reports, symptoms exhibited by COVID-19 patients are nonspecific and its virulence inside the host is getting altered from person to person. In view of this, post-vaccination surveillance plays a crucial role and better results can be achieved by choosing proper biomarkers like nucleic acids or proteins with suitable platforms as shown in Fig. 3 (Udugama et al. 2020).
Nucleic acids biomarkers
Various test kits using nucleic acid as markers are being developed for detection of SARS-CoV-2 among them loop-mediated isothermal amplification (LAMP) and reverse transcription LAMP (RT-LAMP) techniques was successfully tested by various laboratories (Lamb et al. 2020). These come under the category of isothermal amplification techniques because the whole process is conducted at single temperature unlike PCR. LAMP uses DNA polymerase and 4 to 6 primers that bind to distinctive regions of target genome which make the technique highly sensitive. The reaction turns into turbid mixture after encountering with infected patients sample due to the amplification of DNA and results in change of colour based on the dye addition. LAMP is considered as user-friendly approach as it does not require high-end equipments for processing or visualization of test results (Notomi et al. 2000). As an extension, it can be multiplexed during the amplification stage or display stage using barcoded polymeric beads to detect numerous analytes from a single sample/ reaction (Kim et al. 2016). Dunbar (2006) reported usage of fluorophore, which emit signals for detection of captured DNA on bead surface immobilized on the test kits. Clinical specificity can be enhanced to > 90% by employing quantum dot barcodes connected to smart phone for capturing the signal released from the interaction of analyte and bioreceptor (Udugama et al. 2017).
Clustered regularly interspaced short palindromic repeats (CRISPR) is known to be a tool for gene editing but further considering the potentiality of the technique it is used for detection of specific DNA strands in a sample. Broughton et al. (2020) designed DNA endonuclease-targeted CRISPR trans reporter (DETECTR) platform for detection of various strains of coronaviruses using ssDNA. Similarly, SHERLOCK (specific high-sensitivity enzymatic reporter unlocking) detects a particular sequence of viral genes by breaking down and degradation of adjacent ssRNA by the proteins Cas13 (Santiago 2020). Employing of these techniques COVID-19 detection has helped in tracking down the virus with high throughput testing.
Protein biomarkers
Viral protein antigens as well as antibodies developed against SARS-CoV-2 are being used to diagnose COVID-19. Because of variations in viral load during the course of infection it is difficult to detect the viral proteins. For instance, the salivary viral load was highest in the first week of infection which showed gradual decline with time (To et al. 2020). On the other hand generation of antibodies against viral proteins takes a long time for detecting SARS-CoV-2. The major challenge in developing an accurate serological test for SARS-CoV-2 is the cross-reactivity of the SARS-CoV-2 antibodies against other corona viruses. Zhang et al. (2020) detected IgG and IgM from serum of COVID-19 patient using an enzyme-linked immunosorbent assay (ELISA). In this test they used Rp3 nucleocapsid protein of SARS-CoV-2 whose amino acid sequence showed 90% homology with the other SARS-related viruses.
Single Molecule Protein Detection (SIMOA) acts as a powerful tool that helps the researchers to understand the immune response to COVID-19 through antibody response even at early stages of the disease with high resolution (Udugama et al. 2020). In addition, rapid antigen test is intended for qualitative detection of a specific antigen of SARS-CoV-2 present in human nasopharynx. Healthcare professionals collect nasopharyngeal swab from the patient.This test has a sensitivity and a specificity of 99.68% and 96.52% respectively (Udugama et al. 2020).
Besides, there may be several other protein or cellular markers that can be used for detection of SARS-CoV-2. Guan et al. (2020) observed elevated levels of D-dimer, serum ferritin, interleukins, and C-reactive protein; and low levels of leukocytes, lymphocytes and blood platelets. As these biomarkers show abnormality in other illnesses, they are used along with antibodies to improve specificity in multiplex tests. In addition, a point-of-care approach reffered as lateral flow antigen detection is being developed for diagnosing COVID-19. Microfluidics-based smartphone was developed to detect antibodies against several sexually transmitted diseases and the same technology can be adopted for detecting proteins and RNA of SARS-CoV-2 (Laksanasopin et al. 2015; Mahapatra and Chandra 2020; Chandra 2020).
Conclusion
The SARS-CoV-2 infection has created havoc affecting 2,70,79,037 persons and caused death for 8,83,934 worldwide (COVID-19 coronavirus pandemic 2020). Anti-viral drugs and immune-based therapies to treat this deadly virus were successful to some extent but real safety can be achieved only by vaccinating everyone. Several vaccine candidates have reached clinical trials and hopefully, some potent vaccines will be ready for administration in the next couple of months. Besides, variations in virulence among different individuals are questioning the efforts made towards a universal vaccine. The design and development of theranostic vaccines can resolve all such issues. Moreover, the implementation of post-vaccination surveillance could help the researchers to resolve unexpected adverse risks caused due to the immunization process.
References
Ahmed SF, Quadeer AA, McKay MR (2020) Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses 12:254
Angeletti S, Benvenuto D, Bianchi M, Giovanetti M, Pascarella S, Ciccozzi M (2020) COVID-2019: the role of the nsp2 and nsp3 in its pathogenesis. J Med Virol 92:584–588
AstraZeneca (2020) COVID-19 Vaccine enters phase 2/3 clinical trail (2020) https://www.fiercebiotech.com/biotech/astrazeneca-s-covid-19-vaccine-enters-phase-2-3. Accessed 3 Sep 2020
Bartlett G, Antoun J, Zgheib NK (2012) Theranostics in primary care: pharmacogenomics tests and beyond. Expert Rev MolDiagn 12:841–855
Benne N, van Duijn J, Kuiper J, Jiskoot W, Slutter B (2016) Orchestrating immune responses: How size, shape and rigidity affect the immunogenicity of particulate vaccines. J Control Release 234:124–134
Broughton JP, Deng X, Yu G, Fasching CL, Servellita V, Singh J, Miao X, Streithorst JA, Granados A, Sotomayor-Gonzalez A, Zorn K (2020) CRISPR–Cas12-based detection of SARS-CoV-2. Nat Biotechnol 16:1–5
Buonaguro L, Tagliamonte M, Tornesello ML, Buonaguro FM (2020) SARS-CoV-2 RNA polymerase as target for antiviral therapy. J Transl Med 18:1–8
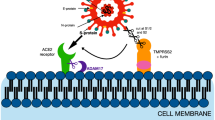
Chan KK, Dorosky D, Sharma P, Abbasi SA, Dye JM, Kranz DM, Herbert AS, Procko E (2020) Engineering human ACE2 to optimize binding to the spike protein of SARS coronavirus 2. Science 369:1261–1265
Chandra P (2020) Miniaturized label-free smartphone assisted electrochemical sensing approach for personalized COVID-19 diagnosis. Sens Int 1:100019
Chávez V (2019) Sources of pre-analytical, analytical and post-analytical errors in the microbiology laboratory. Accurate results in the clinical laboratory. Elsevier, Amsterdam, pp 377–384
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Yu T (2020a) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395:507-513
Chen WH, Tao X, Peng BH, Pollet J, Strych U, Bottazzi ME, Hotez PJ, Lustigman S, Du L, Jiang S, Tseng CTK (2020b) Yeast-expressed SARS-CoV recombinant receptor-binding domain (RBD219-N1) formulated with alum induces protective immunity and reduces immune enhancement. bioRxiv. https://doi.org/10.1101/2020.05.15.098079
Corman VM, Landt O (2020) Detection of 2019-nCoV by RealTime RT-PCR. Euro Surveill 25:23
COVID-19 Coronavirus Pandemic (2020). https://www.worldometers.info/coronavirus/. Accessed 6 Sep 2020
Dang A, Vallish BN, Dang S (2020) Hydroxychloroquine and remdesivir in COVID-19: a critical analysis of recent events. Indian J Med Ethics. https://doi.org/10.20529/IJME.2020.068
Dhama K, Sharun K, Tiwari R, Dadar M, Malik YS, Singh KP, ChaicumpaW, (2020) COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum VaccImmunother. https://doi.org/10.1080/21645515.2020.1735227
Dunbar SA (2006) Applications of LuminexxMAP technology for rapid, high-throughput multiplexed nucleic acid detection. ClinChimActa 363:71–82
Gao J, Tian Z, Yang X (2020a) Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends 14:72-73
Gao Q, Bao L, Mao H, Wang L, Xu K, Yang M, Li Y, Zhu L, Wang N, Lv Z, Gao H (2020b) Development of an inactivated vaccine candidate for SARS-CoV-2. Science. https://doi.org/10.1126/science.abc1932
Gautret P, Lagier JC, Parola P, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Dupont HT, Honoré S (2020) Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 50:105949
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DS, Du B (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382:1708–1720
Hao J, Ren J, Wu Q, HaoY SH, Ning N, Ding D (2017) Identifying factors associated with risk assessment competencies of public health emergency responders. IntEnv Res Pub He 14:597
Holwerda M, Vkovski P, Wider M, Thiel V, Dijkman R (2020) Identification of five antiviral compounds from the Pandemic Response Box targeting SARS-CoV-2. bioRxiv. https://doi.org/10.1101/2020.05.17.100404
Humphreys IR, Sebastian S (2018) Novel viral vectors in infectious diseases. Immunology 153:1–9
Iwen PC, Karen L, Stiles MA (2020) Safety considerations in the laboratory testing of specimens suspected or known to contain the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Am J ClinPathol 153:567–570
Janani M, Venkatesh DN (2019) Clinical evaluation of vaccines. J Pharm Sci Res 11:1775–1780
Jiang S, Bottazzi ME, Du L, Lustigman S, Tseng CTK, Curti E, Jones K, Zhan B, Hotez PJ (2012) Roadmap to developing a recombinant coronavirus S protein receptor-binding domain vaccine for severe acute respiratory syndrome. Expert Rec Vaccines 11:1405–1413
Karabin NB, Allen S, Kwon HK, Bobbala S, Firlar E, Shokuhfar T, Shull KR, Scott EA (2018) Sustained micellar delivery via inducible transitions in nanostructure morphology. Nat Commun 9:1–3
Karimuribo ED, Mutagahywa E, Sindato C, Mboera L, Mwabukusi M, Njenga MK, Teesdale S, Olsen J, Rweyemamu M (2017) A smartphone app (AfyaData) for innovative one health disease surveillance from community to national levels in Africa: intervention in disease surveillance. JMIR Public Health Surveill 3:e94
Kim J, Biondi MJ, Feld JJ, Chan WCW (2016) Clinical validation of quantum dot barcode diagnostic technology. ACS Nano 10:4742–4753
Kim YC, Dema B, Reyes-Sandoval A (2020) COVID-19 vaccines: breaking record times to first-in-human trials. npj Vaccines 5:1–3
Laksanasopin T, Guo TW, Nayak S, Sridhara AA, Xie S, Olowookere OO, Cadinu P, Meng F, Chee NH, Kim J, Chin CD (2015) A smartphone dongle for diagnosis of infectious diseases at the point of care. SciTransl Med 7(273):273re1
Lamb LE, Bartolone SN, Ward E, Chancellor MB (2020) Rapid detection of novel coronavirus (COVID19) by reverse transcription-loop-mediated isothermal amplification. SSRN J. https://doi.org/10.2139/ssrn.3539654
Lau SK, Chan JF (2015) Coronaviruses: emerging and re-emerging pathogens in humans and animals. Virol J 12:209
Le TT, Andreadakis Z, Kumar A, Roman RG, Tollefsen S, Saville M, Mayhew S (2020) The COVID-19 vaccine development landscape. Nat Rev Drug Discov 19:305–306
Liu Z, Xiao X, Wei X, Li J, Yang J, Tan H, Zhu J, Zhang Q, Wu J, Liu L (2020) Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2. J Med Virol. https://doi.org/10.1002/jmv.25726
Lu S (2020) Timely development of vaccines against SARS-CoV-2. Emerg Microbes 9:542–544
Mahapatra S, Chandra P (2020) Clinically practiced and commercially viable nanobio engineered analytical methods for COVID-19 diagnosis. BiosensBioelectron 165:112361
Menachery VD, Gralinski LE, Mitchell HD, Dinnon KH, Leist SR, Yount BL, McAnarney ET, Graham RL, Waters KM, Baric RS (2018) Combination attenuation offers strategy for live attenuated coronavirus vaccine. J Virol 92:e00710-e718
Miller WB, Torday JS, Baluška F (2019) Biological evolution as defense of self. ProgBiophysMolBiol 142:54–74
Njoroge SW, Nichols JH (2014) Risk management in the clinical laboratory. Ann Lab Med 34:274–278
Nordeng H, Ystrøm E, Einarson A (2010) Perception of risk regarding the use of medications and other exposures during pregnancy. Eur J Clin Pharmacol 66:207–214
Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hasen T (2000) Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 28:e63–e63
Pagliusi S, Jarrett S, Hayman B, Kreysa U, Prasad SD, Reers M, Thai PH, Wu K, Zhang YT, Baik YO, Kumar A (2020) Emerging Manufacturers engagements in the COVID-19 vaccine research, development and supply. Vaccine 38:5418–5423
Ramaiah A, Arumugaswami V (2020) Insights into cross-species evolution of novel human coronavirus 2019-nCoV and defining immune determinants for vaccine development. bioRxiv. https://doi.org/10.1101/2020.01.29.925867
Robert-Guroff M (2007) Replicating and non-replicating viral vectors for vaccine development. CurrOpinBIotech 18:546–556
Rothan HA, Byrareddy SN (2020) The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun 109:102433
Santiago I (2020) Trends and innovations in biosensors for COVID-19 mass testing. ChemBioChem 21:1–11
Shen Z, Xiao Y, Kang L, MaW SL, Zhang L, Zhou Z, Yang J, Zhong J, Yang D, Guo L (2020) Genomic diversity of SARS-CoV-2 in Coronavirus Disease 2019 patients. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa203
Shieber JC (2020) Codagenix raises $20 million for a new flu vaccine and other therapies. Tech Crunch. https://techrunch.com/2020/01/13/codagenix-raises-20-million-for-a-new-flu-vaccine-and-othertherapies/. Accessed 30 May 2020
Smith TR, Patel A, Ramos S, Elwood D, Zhu X, Yan J, Gary EN, Walker SN, Schultheis K, Purwar M, Xu Z (2020) Immunogenicity of a DNA vaccine candidate for COVID-19. Nat Commun 11:1–13
Swadling L, Klenerman P, Barnes E (2013) Ever closer to a prophylactic vaccine for HCV. Expert opinbiolther 13:1109–1124
Ta L, Gosa L, Nathanson DA (2019) Biosafety and biohazards: understanding biosafety levels and meeting safety requirements of a biobank. In: Yong W (ed) Biobanking. Methods in molecular biology. Humana Press, New York
Tetro JA (2020) Is COVID-19 receiving ADE from other coronaviruses? Microb infect 22:72–73
To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC, Yip CC, Cai JP, Chan JM, Chik TS, Lau DP (2020) Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 20:565–574
Udugama B, Kadhiresan P, Samarakoon A, Chan WCW (2017) Simplifying assays by tableting reagents. J Am ChemSoc 139:17341–17349
Udugama B, Kadhiresan P, Kozlowski HN, Malekjahani A, Osborne M, Li VY, Chen H, Mubareka S, Gubbay JB, Chan WC (2020) Diagnosing COVID-19: the disease and tools for detection. ACS Nano 14:3822–3835
Vellozzi C, Burwen DR, Dobardzic A, Ball R, Walton K, Haber P (2009) Safety of trivalent inactivated influenza vaccines in adults: background for pandemic influenza vaccine safety monitoring. Vaccine 27:2114–2120
Wang C, Liu Z, Chen Z, Xin H, Mengyuan X, Tengfei H, Zhenhua Z (2020) The establishment of reference sequence for SARS-CoV-2 and variation analysis. J Med Virol 92:667–674
Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G (2020) Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 30:269–271
Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q, Hu Y (2020) Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 395:1569–1578
Weinberger B (2018) Vaccines for the elderly: current use and future challenges. Immun Ageing 15:3
WHO (2020a) WHO, UN Foundation and Partners Launch First-of-Its-Kind COVID-19 Solidarity Response Fund. https://www.who.int/news-room/detail/13-03-2020-who-un-foundation-and-partners-launch-firstof-its-kind-covid-19-solidarity-response-fund Accessed 15 June 2020
WHO (2020b) DRAFT landscape of COVID-19 candidate vaccines. World. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines. Accessed 3 Sep 2020
Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, Tian JH, Pei YY, Yuan ML (2020) A new coronavirus associated with human respiratory disease in China. Nature 579:265–269
Zhang W, Du RH, Li B, Zheng XS, Yang XL, Hu B, Wang YY, Xiao GF, Yan B, Shi ZL, Zhou P (2020) Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect 9:386–389
Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–273
Zhu FC, Li YH, Guan XH, Hou LH, Wang WJ, Li JX, Wu SP, Wang BS, Wang Z, Wang L, Jia SY (2020) Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 395:1845–1854
Zuniga S, Sol I, Moreno JL, Sabella P, Plana-Durán J, Enjuanes L (2007) Coronavirus nucleocapsid protein is an RNA chaperone. Virology 35:15–227
Acknowledgement
The authors are grateful to Director, ICAR-IISS, Mau; Management, VFSTR, India, and Dean, COA, Pasighat and VC, CAU, Imphal for extending their kind support.
Funding
No financial assistance was received either from government or any private agency for the present work.
Author information
Authors and Affiliations
Contributions
NSSK: Resources, Writing-original draft, ADC: Conceptualization, SPJK: review, SR: editing, Mahesh Kumar: editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Human and animal rights
This article does not contain any studies with human or animal subjects.
Rights and permissions
About this article
Cite this article
Sampath Kumar, N.S., Chintagunta, A.D., Jeevan Kumar, S.P. et al. Immunotherapeutics for Covid-19 and post vaccination surveillance. 3 Biotech 10, 527 (2020). https://doi.org/10.1007/s13205-020-02522-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-020-02522-9